What if a single hormone could control appetite in two distinct ways? A University College London professor reveals how GLP-1’ s dual role in the brain and gut could transform the treatment of obesity.
For decades, researchers have explored how the body regulates hunger and fullness. A central player in this system is glucagon-like peptide 1 (GLP-1) , a hormone originally known for its role in regulating blood sugar. But as Professor Stefan Trapp, an expert in autonomic neuroscience and metabolic diseases at University College London, explains, GLP-1’ s role in appetite is far more complex—it originates from two distinct sources (the gut and the brain) and exerts its effects through distinct mechanisms.
Trapp, with nearly 20 years of experience studying how the brain controls eating behavior, has helped change the scientific narrative. His groundbreaking research on proglucagon (PPG) neurons and the role of the blood-brain barrier in restricting GLP-1 access to the brain is redefining our understanding of obesity, appetite, and drug development.
The Gut-Brain Axis: Challenging the Old GLP-1 Model
The original theory was simple: food enters the gut → the gut releases GLP-1 → GLP-1 enters the brain via the bloodstream → GLP-1 induces the release of more GLP-1 in the brain → appetite is suppressed.
But the lab discovered a key flaw: the preproglucagon (PPG) neurons in the brainstem that produce GLP-1 lack receptors for circulating GLP-1. This means that the gut-derived hormone GLP-1 cannot directly stimulate these brain cells. Instead, the central GLP-1 system operates independently, driven by physiological signals such as gastric distension and neural input, rather than circulating GLP-1.
This discovery has prompted further investigation into the distinct effects of GLP-1 in the periphery and central nervous system. Trapp emphasized the fundamental difference between the actions of hormones and neurotransmitters. “ Neurotransmitters are released in a very specific part of the brain, ” he explained. “ They act at specific synapses and cover only a very small area, so they produce signals that are very precise in both time and space. ”
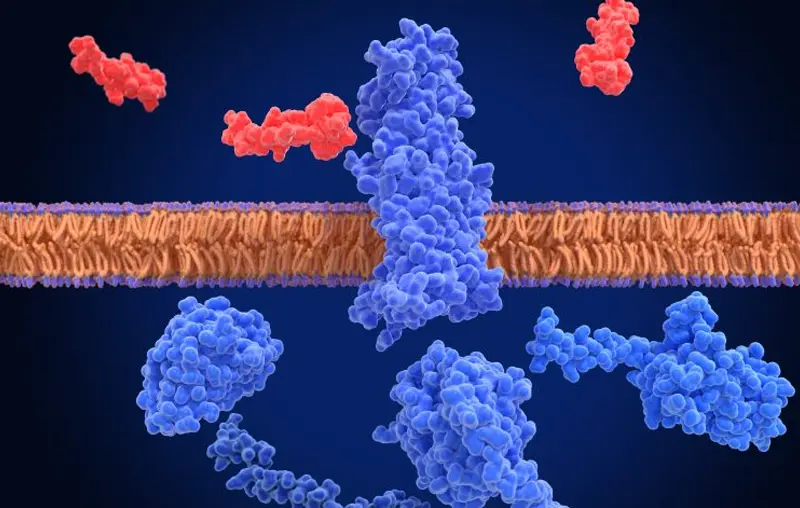
Peripheral GLP-1 is a hormone released into the bloodstream after a meal and can simultaneously reach any GLP-1 receptor throughout the body, thereby exerting a broader range of effects. Its primary action is to enhance insulin release in response to elevated blood glucose levels, a mechanism already used in the treatment of type 2 diabetes. In contrast, GLP-1 produced in the brain is a neurotransmitter. It is released at specific synaptic sites within the brain, delivering spatially precise signals. This means that GLP-1 can be released in one brain region (such as the hypothalamus) without affecting another (such as the amygdala) . This localized action allows for fine-tuning of diverse brain functions related to appetite.
Trapp also emphasized the crucial barrier between the bloodstream and the brain—the blood-brain barrier (BBB) . “It protects our brain from a wide range of substances, chemicals, and bacteria, ” Trapp said. Peptides like GLP-1 have difficulty crossing this barrier, challenging the assumption that peripheral GLP-1 can easily affect brain function. While crucial for brain health, this protective mechanism poses significant challenges for drug delivery, hindering the development of therapies targeting the central GLP-1 pathway.
This prompted Trapp’ s team to explore whether the brain and peripheral GLP-1 systems might operate independently. Their findings increasingly support this idea, suggesting that effective treatments may need to mobilize the two systems in different ways.